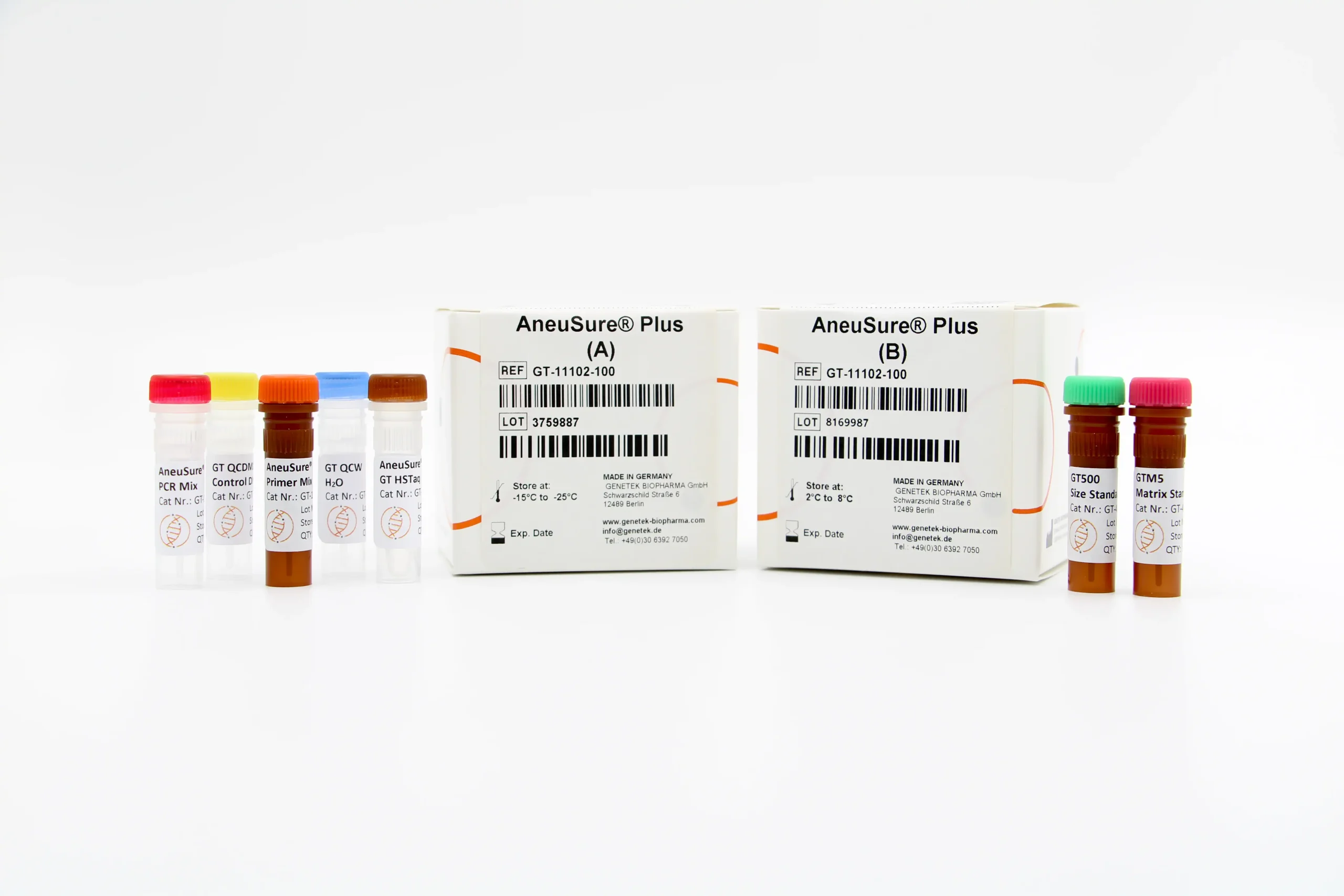
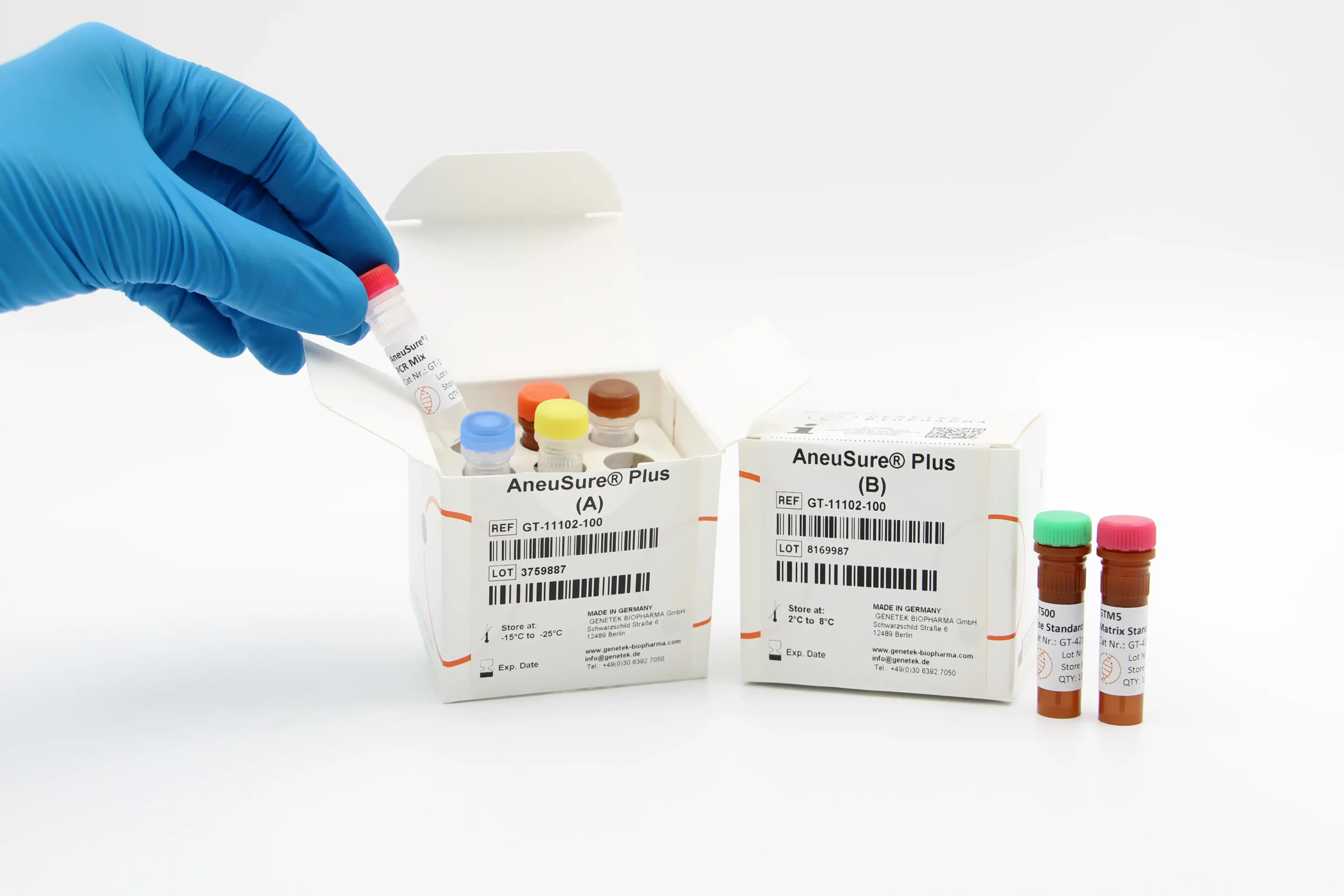
AneuSure® Plus
AneuSure® Plus Kit is a QF-PCR kit consisting of 26 markers for rapid prenatal diagnosis of chromosomal aneuploidy and 2 markers for 5q SMA detection. The aneuploidy markers are for chromosomes 21 (Down Syndrome or +21), 18 (Edwards Syndrome or +18), 13 (Patau Syndrome or +13) as well as 47, XXY (Klinefelter Syndrome), 47, XXX (Triple X Syndrome), 45, X (Turner Syndrome) and other sex chromosomes. The AneuSure® Plus QF-PCR STR markers are distributed across autosomal chromosomes 21, 18 and 13 and sex chromosomes X & Y. The segmental duplication 7X marker is included in the kit for the differentiation of X chromosome monosomy from homozygosity (i.e., it quantifies chromosome X for more accurate detection of Turner Syndrome).
AneuSure® Plus kit also includes markers for the detection of 5q deletion or conversion on exon 7 of SMN1 and SMN2. Spinal Muscular Atrophy (SMA) is one of the most prevalent neuromuscular disorders. The disease is inherited as an autosomal recessive disorder.
AneuSure® Plus Premium Features
- Simultaneous detection of common chromosomal aneuploidies and deletion or conversion of exon 7 of SMN1 gene cause of SMA;
- Multiplex analysis of 28 markers in one reaction;
- Rapid diagnostic follow-up after NIPT tests;
- Accurate detection of Turner Syndrome;
- Applicable to a variety of DNA sources – amniotic fluid (AF), chorionic villus (CVS), fetal tissue, etc.;
- Can be used by any of Genetek Direct PCR products like GT DBC, GT DBC Blue, as well as direct amplification of Blood, Amniotic Fluid and other cultured cells, Chorionic Villi and other tissues suing these lysis buffers (i.e., GT BLB, GT AFLB and GT CVLB);
- Easy to use mix
For research use only. Not for use in diagnostic procedures.
Markers
D21S1809, D21S1446, D21IFNAR, D21S1414, D21S1442, D21S1411, D18S390, D18S391, D18S1002, D18S535, D18-GATA178F11, D13S325, D13S252, D13S634, D13S258, D13S797, DXS7132, HPRT, DXS6803, 7X, DX-TATC 13.3, DXS981, AMXY, SRY, Y/X b, DYS437, SMN1 and SMN2 totaling 28 markers.
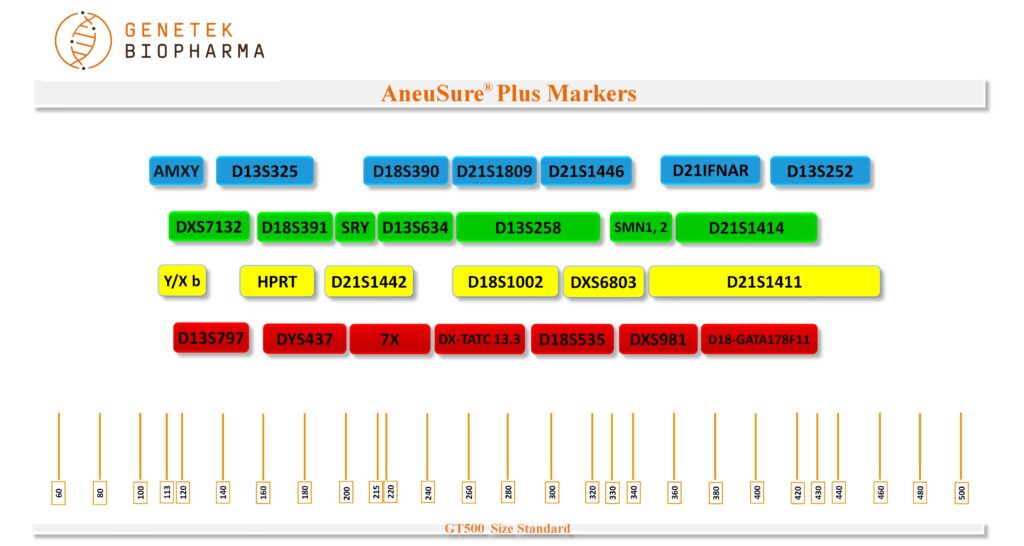
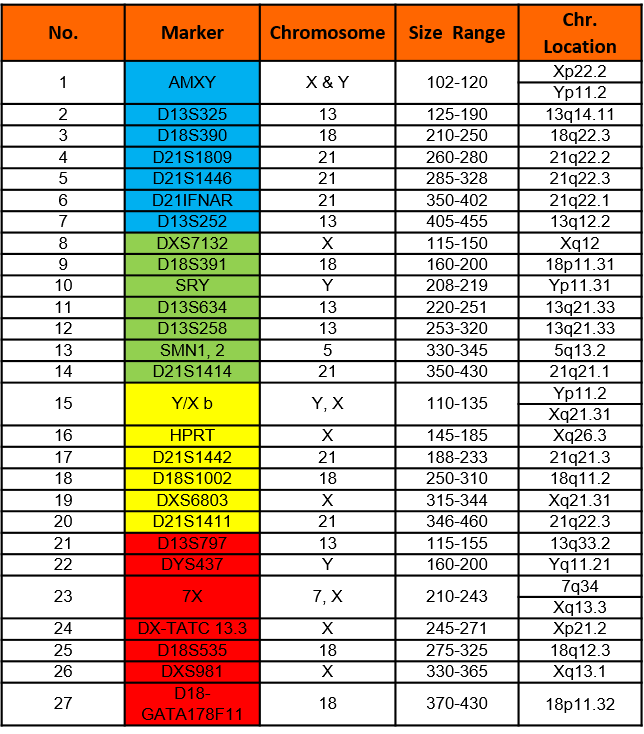
Chromosome Locations
Table below shows markers in the AneuSure® Plus Kit. Names, dye used, size range and chromosome locations are shown.
Sample Profile
| Quality Control DNA | Download |
| Down Syndrome (+21) | Download |
| Edward Syndrome (+18) | Download |
| Patau Syndrome (+13) | Download |
| Triploidy 69 | Download |
| Turner Syndrome (XO) | Download |
| Klinefelter Syndrome (XXY) | Download |
| Triple X Syndrome (XXX) | Download |
| Affected with SMA | Download |
Comparison Table
The table shows number of markers for each Genetek QF-PCR kit. There are 26 markers in the AneuSure, 28 in the AneuSure Plus, 32 in the AneuSure Extra, 34 in AneuSure Extra Plus, 32 in AneuSure Max and 34 in AneuSure Max Plus kits. There are 7 segmental duplications in the AneuSure Extra and AneuSure Extra Plus. There are 2 segmental duplication and 5 additional STR markers in AneuSure Max and AneuSure® Max Plus kits. The availability of the above-mentioned kits provide medical geneticists with many powerful options to choose from, considering the requirements of each case. Our innovative inclusion of SMA detection power (by addition of SMN1 and SMN2 exon 7 deletion and conversion in AneuSure Plus, AneuSure Extra Plus and AneuSure Max Plus kits) creates a considerable advantage for any laboratory to detect fetuses who may be normal for aneuploidy but affected with a deadly and costly disease like SMA.
| Features | AneuSure (QF-PCR Kit) | AneuSure® Extra (QF-PCR Kit) | AneuSure® Max (QF-PCR Kit) | AneuSure® Plus (QF-PCR Kit+ SMA) | AneuSure® Extra Plus (QF-PCR Kit+ SMA) | AneuSure® Max Plus (QF-PCR Kit+ SMA) |
| Total Markers | 26 | 32 | 32 | 28 | 34 | 34 |
| Y markers | 2 | 2 | 2 | 2 | 2 | 2 |
| X markers | 6 | 7 | 7 | 6 | 7 | 7 |
| X/Y markers | 2 | 2 | 2 | 2 | 2 | 2 |
| Chr. 21 markers | 6 | 8 | 8 | 6 | 8 | 8 |
| Chr. 18 markers | 5 | 8 | 7 | 5 | 8 | 7 |
| Chr. 13 markers | 5 | 6 | 7 | 5 | 6 | 7 |
| Segmental Duplication | 1 | 7 | 2 | 1 | 7 | 2 |
| SMA Detection | No | No | No | ✓ | ✓ | ✓ |
| 5- or 6-dye system | 5 | 6 | 6 | 5 | 6 | 6 |
| Single-tube multiplex | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| ABI GA Systems | 3130/xl3500/xLSeqStudio | 3500/xLSeqStudio | 3500/xLSeqStudio | 3130/xl3500/xLSeqStudio | 3500/xLSeqStudio | 3500/xLSeqStudio |
Compatibility
This kit is compatible with 5-dye system of capillary electrophoresis such as with ABI 3130/xl, 3500/xL, SeqStudio Genetic Analyzers with either 30, 50, or 80 capillaries.
This kit can be used on various sources of DNA including DNA extracted from various sources or DNA obtained using any of extraction free (direct) materials from Genetek such as GT AFLB (Amniotic Fluid Lysis Buffer), GT CVLB (Chorionic Villus Lysis Buffer, GT BLB (Blood Lysis Buffer), and filter papers (GT DBC or GT DBC Blue).
Kit Content
The kit contains all the necessary reagents and buffers for multiplex PCR (Multiplex Primer mix, PCR buffer, GT HSTaq DNA Pol in Box A). Also, the kit comes with GT500 size standard as well as GTM5 Matrix Standard for calibrating the Genetic Analyzer (Box B).