AneuSure® Max
AneuSure® Max Kit is a QF-PCR kit consisting of 32 markers for rapid prenatal diagnosis of aneuploidies of chromosomes 21 (Down Syndrome or +21), 18 (Edwards Syndrome or +18), 13 (Patau Syndrome or +13) as well as 47, XXY (Klinefelter Syndrome), 47, XXX (Triple X Syndrome), 45, X (Turner Syndrome) and other sex chromosomes aneuploidies. AneuSure® Max QF-PCR STR markers are distributed across autosomal chromosomes 21, 18, 13, and sex chromosomes (X & Y). Segmental duplication (SD) 7/X and 18/X markers are included in the kit for the differentiation of X chromosome monosomy from homozygosity (i.e., it quantifies chromosome X for more accurate detection of Turner Syndrome and other X chromosome aneuploidies). Additionally, this kit includes five more STR markers to make eight markers on chromosome 21, seven markers for chromosome 18, seven markers for chromosome 13, seven markers for chromosome X, three sex typing markers, totaling 32 markers.
AneuSure® Max Premium Features
- Simultaneous detection of common chromosomal aneuploidies;
- Easy to use mix;
- Multiplex analysis of 32 loci in one reaction eliminate use of extra kits or chromosome specific marker kits, which makes it less expensive and less of lab work;
- Rapid diagnostic follow-up after NIPT test;
- Accurate detection of Turner Syndrome and other X chromosome aneuploidies;
- Applicable to a variety of DNA sources – amniotic fluid (AF), chorionic villus (CVS), fetal tissue, etc.
- Can be used by any of Genetek Direct PCR products like GT DBC, GT DBC Blue, as well as direct amplification of Blood, Amniotic Fluid and other cultured cells, Chorionic Villi and other tissues suing these lysis buffers (i.e., GT BLB, GT AFLB and GT CVLB);
For research use only. Not for use in diagnostic procedures.
Markers
D21S1809, D21S1446, D21IFNAR, D21S1414, D21S1442, D21S1411, D21S1437, D21S1435, D18S390, D18S391, D18S1002, D18S535, D18-GATA178F11, D18S978, D13S325, D13S252, D13S634, D13S258, D13S797, D13S628, D13S742, DXS7132, HPRT, DXS6803, DX-TATC 13.3, DXS981, 7/X, 18/X AMXY, SRY, Y/X b and DYS437 totaling 32 markers.
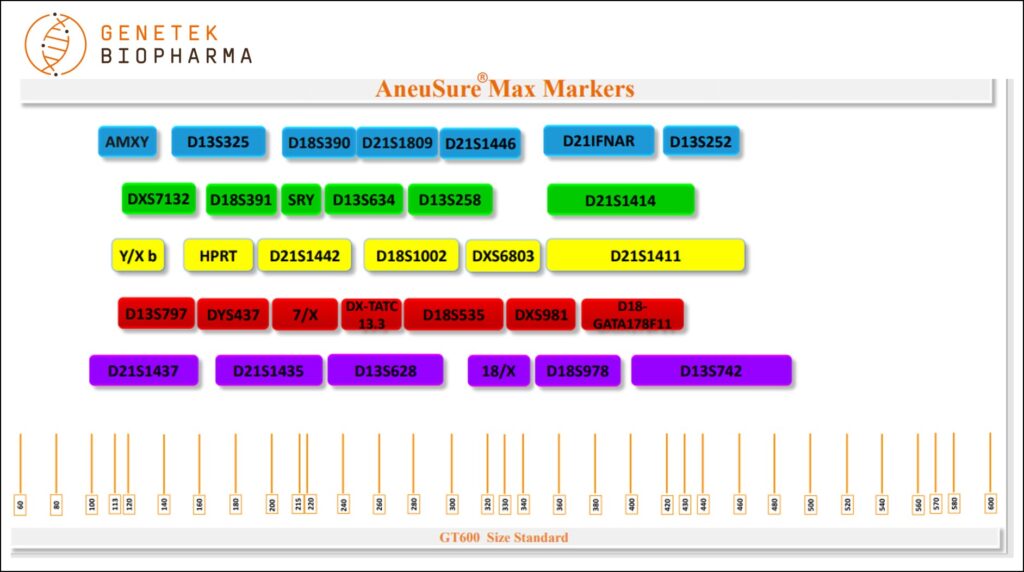
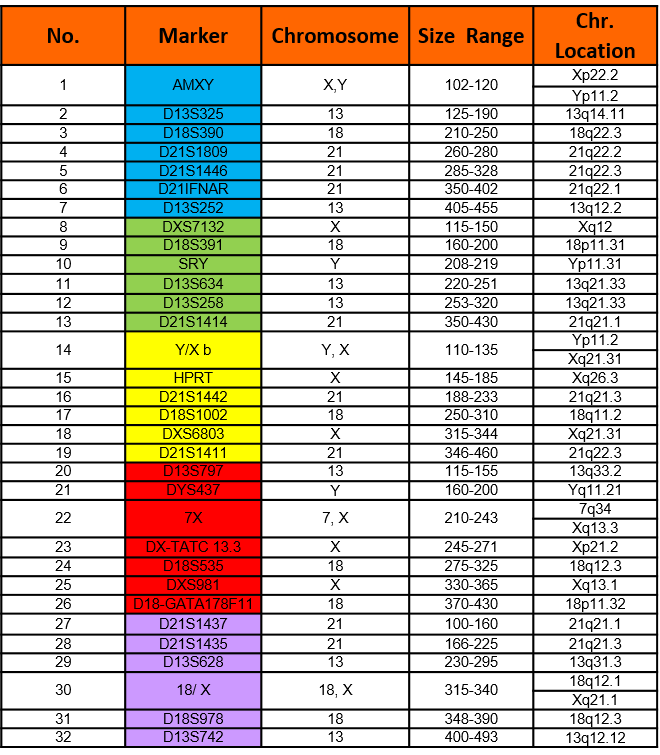
Chromosome Locations
Table below shows markers in the AneuSure® Max Kit. Names, dye used, size range and chromosome locations are shown.
Sample Profile
| Quality Control DNA | Download |
| Down Syndrome (+21) | Download |
| Edward Syndrome (+18) | Download |
| Patau Syndrome (+13) | Download |
| Turner Syndrome (XO) | Download |
| Triple X Syndrome (XXX) | Download |
Comparison Table
The table shows number of markers for each Genetek QF-PCR kit. There are 26 markers in the AneuSure, 28 in the AneuSure Plus, 32 in the AneuSure Extra, 34 in AneuSure Extra Plus, 32 in AneuSure Max and 34 in AneuSure Max Plus kits. There are 7 segmental duplications in the AneuSure Extra and AneuSure Extra Plus. There are 2 segmental duplication and 5 additional STR markers in AneuSure Max and AneuSure® Max Plus kits. The availability of the above-mentioned kits provide medical geneticists with many powerful options to choose from, considering the requirements of each case. Our innovative inclusion of SMA detection power (by addition of SMN1 and SMN2 exon 7 deletion and conversion in AneuSure Plus, AneuSure Extra Plus and AneuSure Max Plus kits) creates a considerable advantage for any laboratory to detect fetuses who may be normal for aneuploidy but affected with a deadly and costly disease like SMA.
| Features | AneuSure (QF-PCR Kit) | AneuSure® Extra (QF-PCR Kit) | AneuSure® Max (QF-PCR Kit) | AneuSure® Plus (QF-PCR Kit+ SMA) | AneuSure® Extra Plus (QF-PCR Kit+ SMA) | AneuSure® Max Plus (QF-PCR Kit+ SMA) |
| Total Markers | 26 | 32 | 32 | 28 | 34 | 34 |
| Y markers | 2 | 2 | 2 | 2 | 2 | 2 |
| X markers | 6 | 7 | 7 | 6 | 7 | 7 |
| X/Y markers | 2 | 2 | 2 | 2 | 2 | 2 |
| Chr. 21 markers | 6 | 8 | 8 | 6 | 8 | 8 |
| Chr. 18 markers | 5 | 8 | 7 | 5 | 8 | 7 |
| Chr. 13 markers | 5 | 6 | 7 | 5 | 6 | 7 |
| Segmental Duplication | 1 | 7 | 2 | 1 | 7 | 2 |
| SMA Detection | No | No | No | ✓ | ✓ | ✓ |
| 5- or 6-dye system | 5 | 6 | 6 | 5 | 6 | 6 |
| Single-tube multiplex | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| ABI GA Systems | 3130/xl3500/xLSeqStudio | 3500/xLSeqStudio | 3500/xLSeqStudio | 3130/xl3500/xLSeqStudio | 3500/xLSeqStudio | 3500/xLSeqStudio |
Compatibility
This kit is compatible with 6-dye system of capillary electrophoresis such as with ABI 3500/xL and SeqStudio Genetic Analyzers from Thermo Fisher.
This kit can be used on various sources of DNA including DNA extracted from various sources or DNA obtained using any of extraction free (direct) materials from Genetek such as GT AFLB (Amniotic Fluid Lysis Buffer), GT CVLB (Chorionic Villus Lysis Buffer, GT BLB (Blood Lysis Buffer), and filter papers (GT DBC or GT DBC Blue).
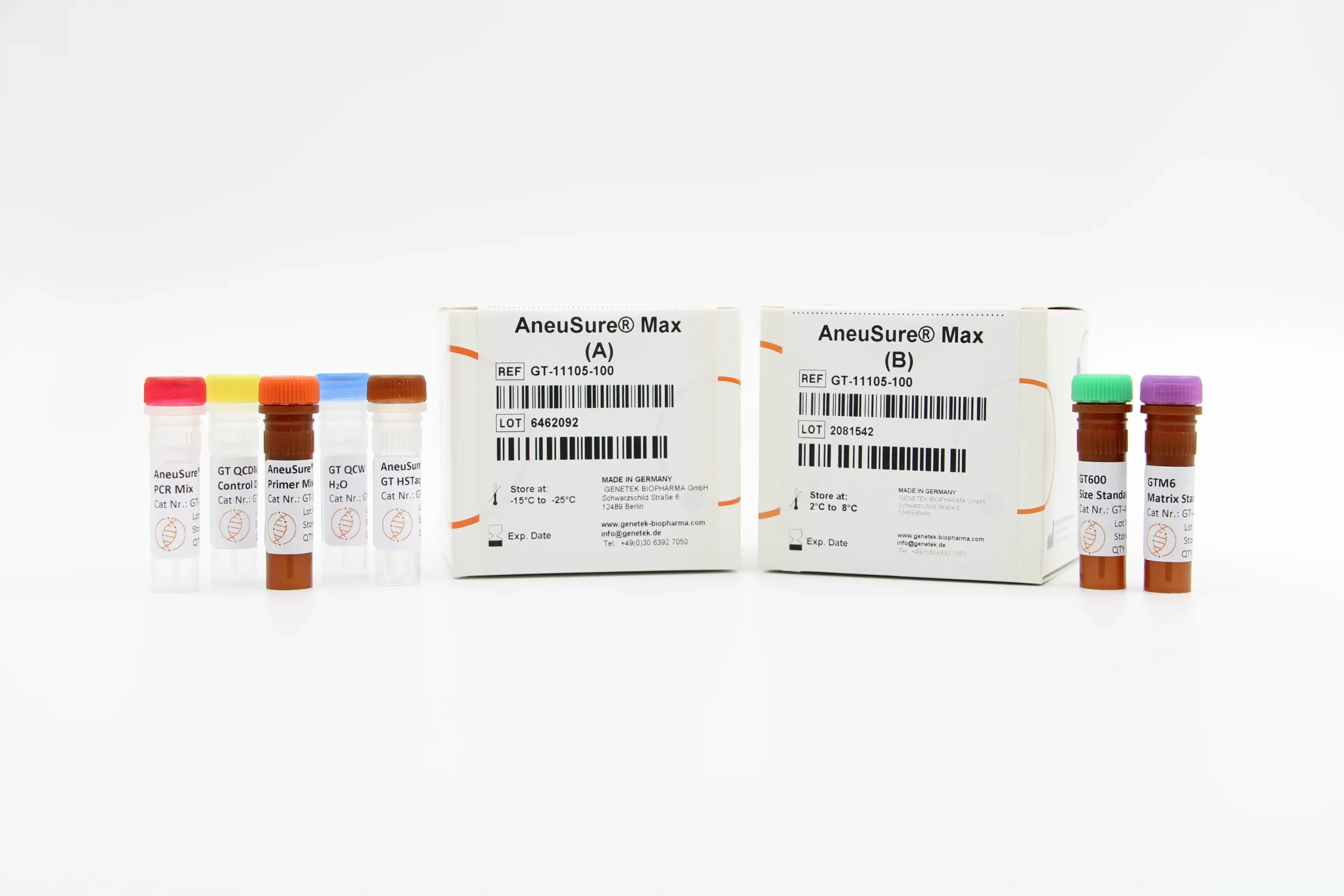
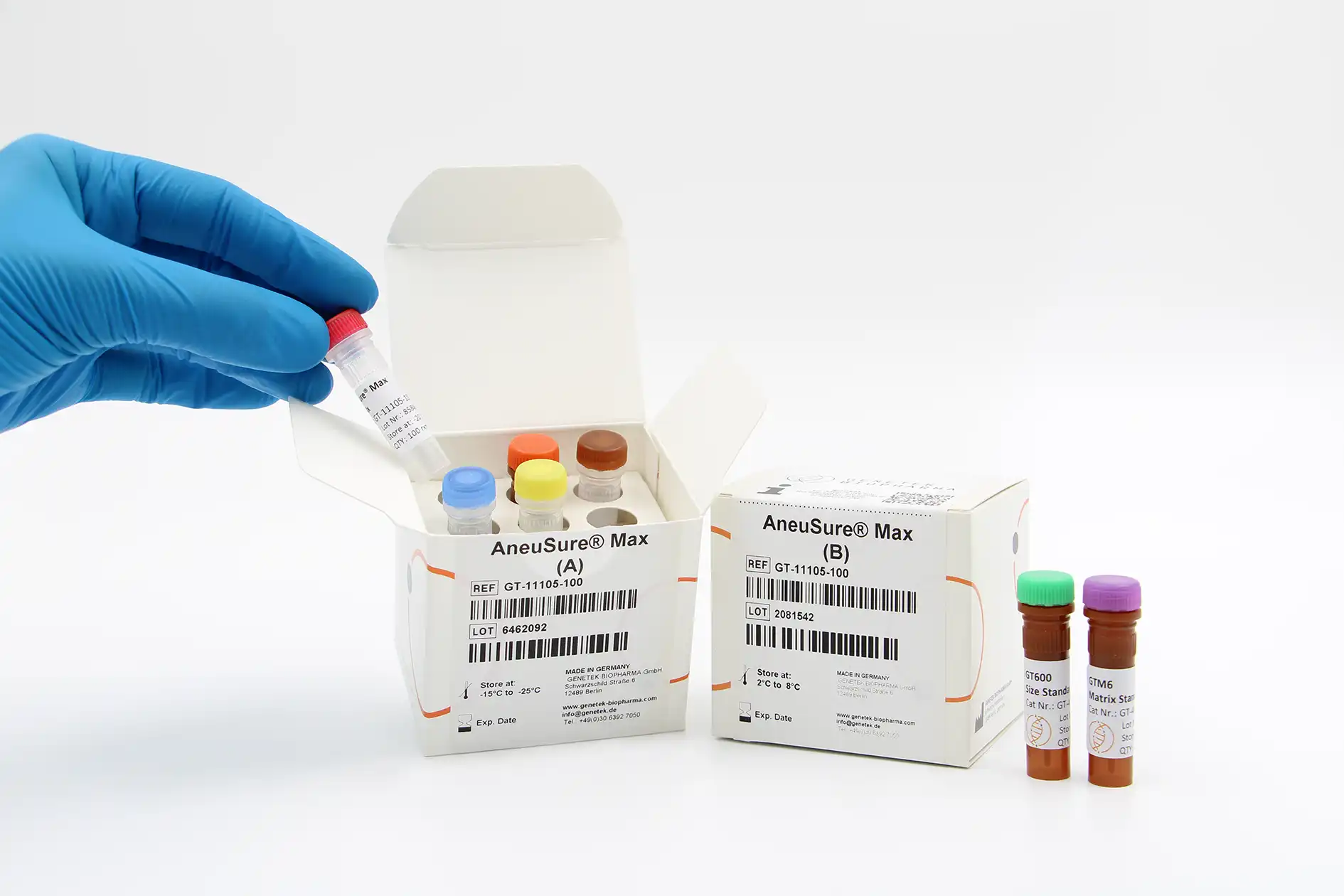
Kit Content
The kit contains all the necessary reagents and buffers for multiplex PCR (Multiplex Primer mix, PCR buffer, GT HSTaq DNA Pol in Box A). Also, the kit comes with GT600 size standard as well as GTM6 Matrix Standard for calibrating the Genetic Analyzer (Box B).