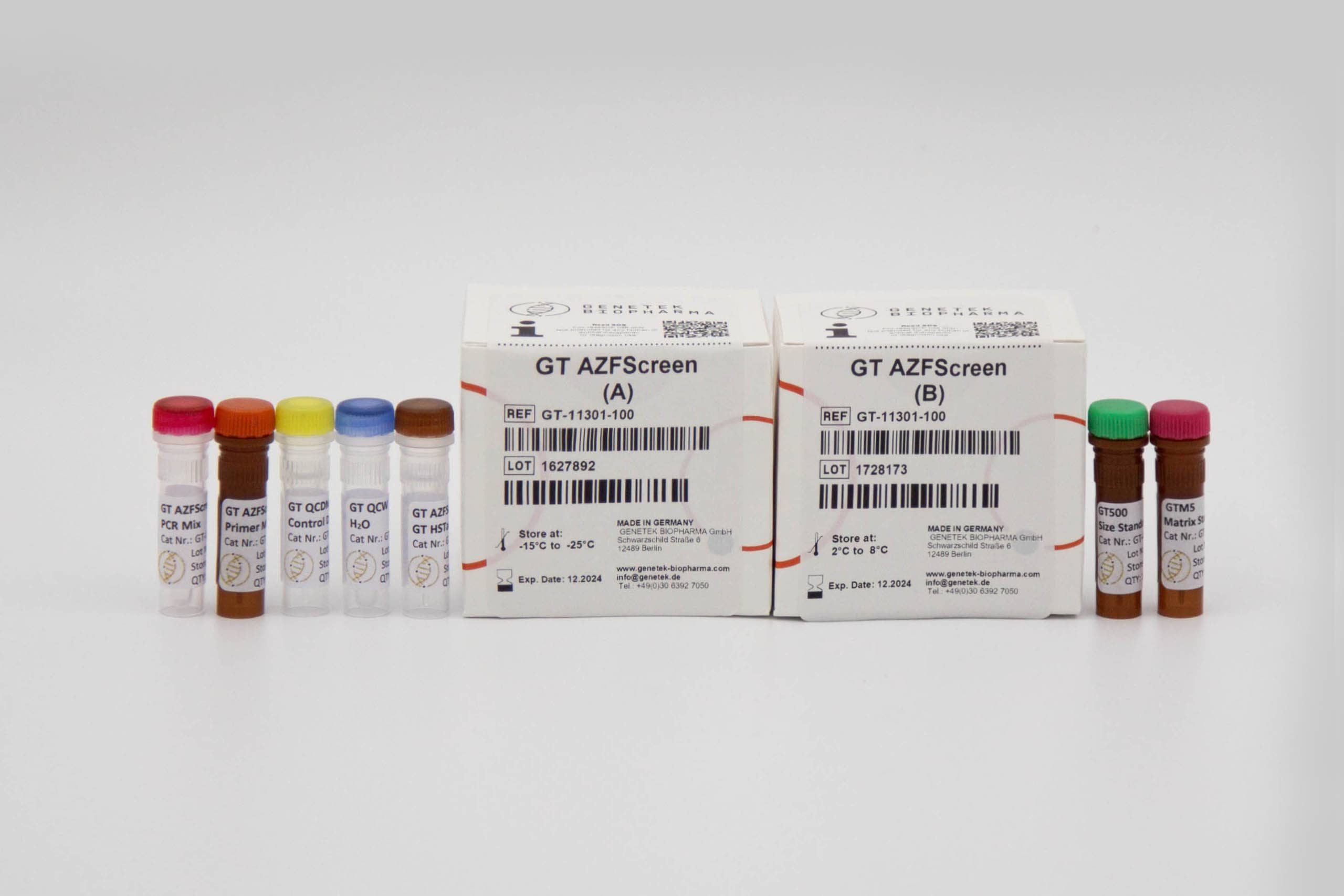
GT AZFScreen
GT AZFScreen kit is an STS-based (Sequence-Tagged Sites) kit for rapid and accurate detection of Y chromosome microdeletions in cases of male infertility. GT AZFScreen kit is developed for the detection of Y chromosome microdeletions, a frequent genetic cause of infertility in men. Infertility is regarded as a critical problem for most couples. It affects 15-20 % of reproductive-age couples worldwide. Male infertility contributes to about 20-30% of these cases, out of which Y chromosome microdeletions cause 5-10 %.
GT AZFScreen kit includes 19 markers (including 16 STS, 2 SD [Segmental Duplication] and 1 STR). Primer design followed European Molecular Genetics Quality Network guidelines.
The GT AZFScreen kit can also detect Klinefelter Syndrome (47, XXY), another cause of male infertility in men. However, we recommend the use of GT AZFScreen Plus for detection of Klinefelter Syndrome. GT AZFScreen kit performance has been validated with extensive testing using Applied Biosystems™ 3500/xL and 3130/xl platforms for detection and analysis on more than 500 samples, either as normal, with Klinefelter Syndrome, deletions of AZFa, AZFb, AZFc, AZFd, or any combination of these deletions.
GT AZFScreen Premium Features
- Multiplex analysis of 19 markers in single reaction which makes it less expensive and less of lab work;
- For rapid and accurate detection of deletions in any of AZF related regions on human Y chromosome as well as markers for Klinefelter Syndrome detection;
- Rapid testing for AZF related infertility in men as well as screening for Klinefelter Syndrome;
- Applicable to a variety of DNA sources including our DNA extraction free materials like blood on filter paper like GT DBC, GT DBC Blue, and GT BLB;
- Easy to use mix and rapid PCR to result;
- Easy to interpret results.
Markers
SY153 (AZFd), SY127 (AZFb), SY157 (AZFc), SY625 (AZFa), X/Y b, SY90, M259 (AZFa), SRY(SY14), SY130 (AZFb), AMXY, SY86 (AZFa), ZFX/Y, SY84 (AZFa), SY254 (AZFc), SY134 (AZFb), SY255 (AZFc), DXS7132, SY131 (AZFb) and SY152(AZFd).
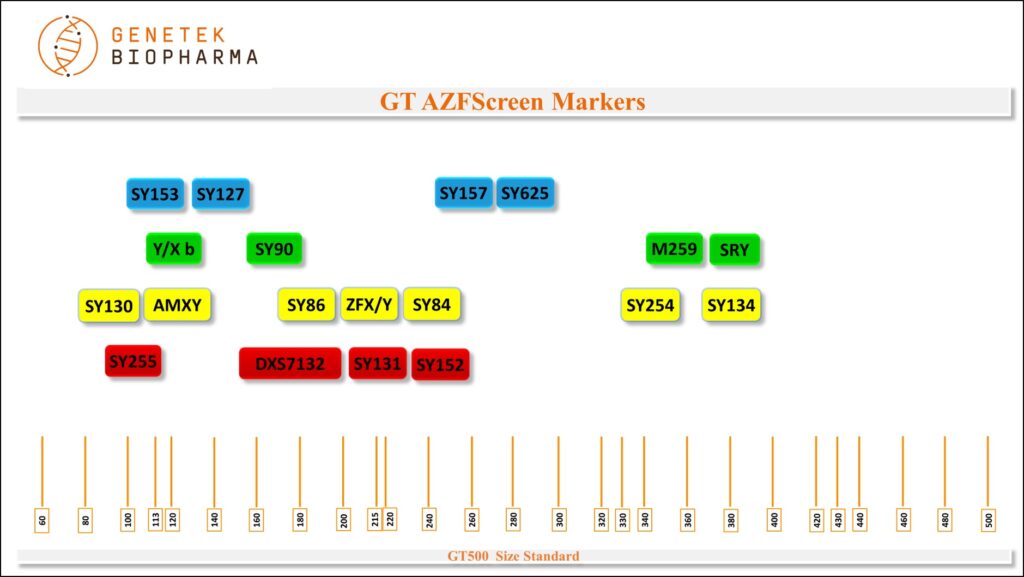
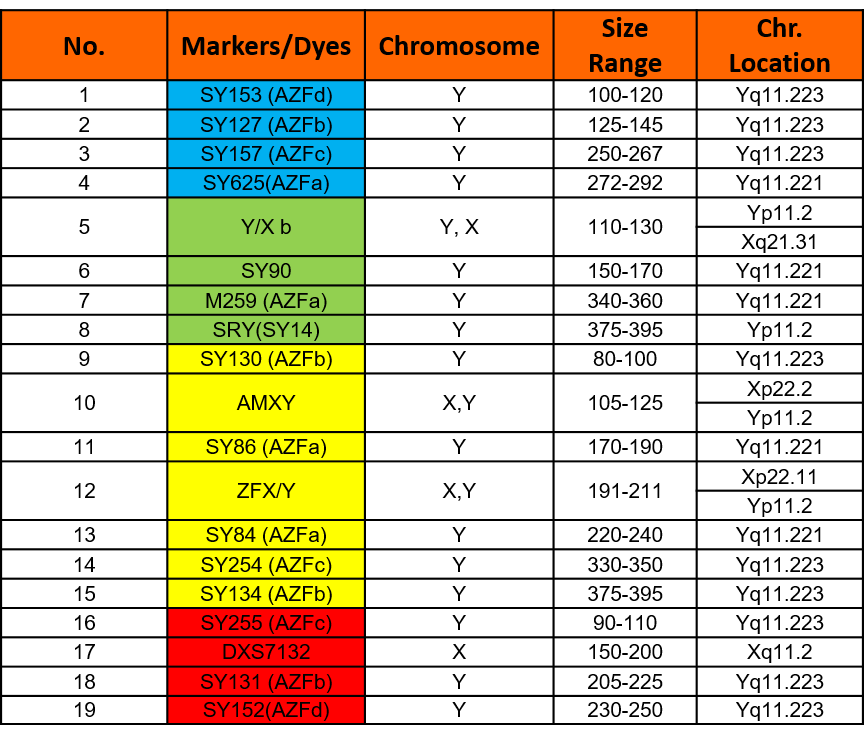
Chromosome Locations
Table below shows markers in the GT AZFSCreen Kit. Names, dye used, size range and chromosome locations are shown.
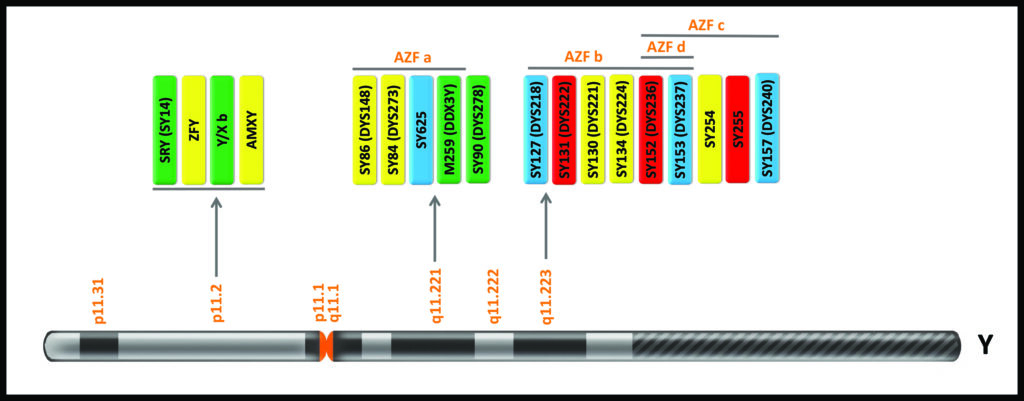
The Y Chromosome locations of the AZF and other markers for the GT AZFScreen kit
Sample Profile
| Quality Control DNA | Download |
| AZFa Microdeletions | Download |
| AZFb Microdeletions | Download |
| Klinefelter Syndrome (XXY) | Download |
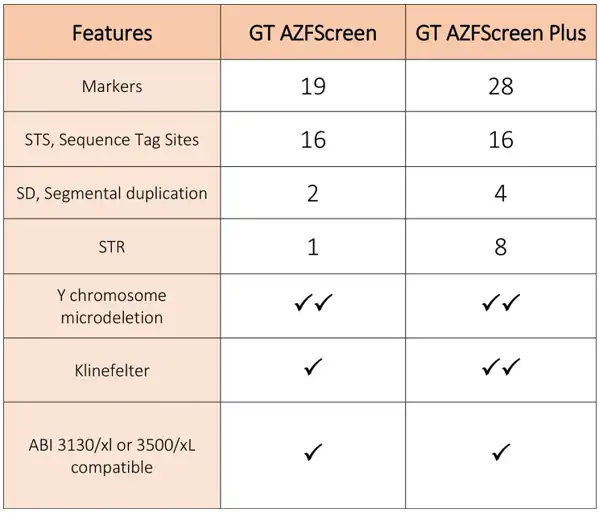
Comparison Table
The table compares Genetek Biopharma’s AZFScreen kits for the detection of deletion in the AZF region of the Y-chromosome in male infertility as well as detection of Klinefelter syndrome.
Compatibility
This kit is compatible with 5-dyes capillary electrophoresis system such as with ABI 3130/xl, 3500/xL , and SeqStudio Genetic Analyzers with either 30, 50, or 80 capillaries.
This kit can be used on various sources of DNA including DNA extracted from various sources or DNA obtained using any of extraction free (direct) materials from Genetek such as GT AFLB (Amniotic Fluid Lysis Buffer), GT CVLB (Chorionic Villus Lysis Buffer, GT BLB (Blood Lysis Buffer), and filter papers (GT DBC or GT DBC Blue).
Kit Content
The kit contains all the necessary reagents and buffers for multiplex PCR (Multiplex Primer mix, PCR buffer, GT HSTaq DNA Pol in Box A). Also, the kit comes with GT500 size standard as well as GTM5 Matrix Standard for calibrating the Genetic Analyzer (Box B).