GT HapScreen® DMD
For more accurate and risk free prenatal diagnosis of DMD/BMD
Duchenne Muscular Dystrophy (DMD) is the most common childhood form of Muscular Dystrophy. It affects 1 in 3500 - 5000 boys. The milder form of the disease is called Becker Muscular Dystrophy (BMD). The disease is inherited as X-linked recessive; so, it is predominantly seen in males. The gene responsible for the disease is called Dystrophin, which is one of the largest genes expanding over 76 exons on the short arm of the X-chromosome. DMD is predominantly caused by deletion or duplication, though point mutation is also seen in about 30% of cases. DMD in about 30% of the cases is caused by a new mutation. This makes the diagnosis even more challenging.
Prenatal diagnosis (PND) for inherited disorders have become a routine practice. Millions of PNDs are performed each year worldwide. Molecular prenatal detection is of crucial importance since most of the PNDs are carried out using this procedure. PND is prone to misdiagnosis for various purposes including maternal cell contamination, sample mix-up, sample contaminated with other samples, gonadal mosaicism, etc. The advent of Next Generation Sequencing (NGS) has enabled us to better detect the molecular basis of many inherited disorders including DMD. Some of these disorders are more prevalent in some parts of the world or some specific ethnicities (such as beta-thalassemia, H-disease, Sickle cell disease, and cystic fibrosis), however; the incidence of DMD/BMD is somewhat similar worldwide.
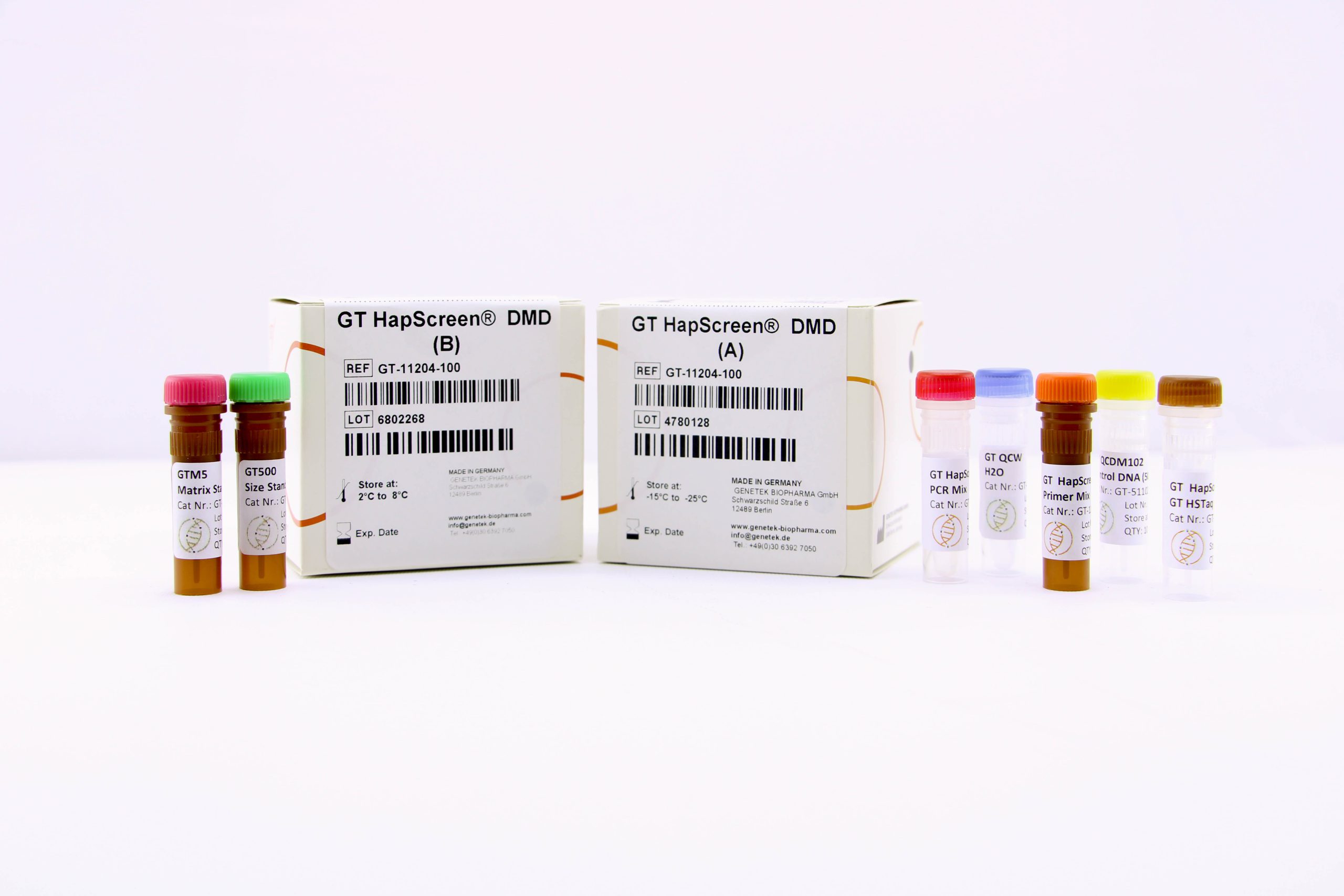
The GT HapScreen® DMD kit has been developed to detect carriers or affected individuals for DMD/BMD. The kit functions based on STR markers and QF-PCR technique to detect carriers as well as fetal samples by linkage or segregation analysis. Use of STR markers makes prenatal diagnosis more accurate, more reliable and less prone to common errors.
Kit components include multiplex PCR primers which are designed flanking the dystrophin gene. This gene is investigated in carrier detection and prenatal diagnosis of Duchenne or Becker Muscular Dystrophies. STR markers designed from 6 regions that cover the upstream, downstream and intron of the dystrophin gene. This kit is optimized to use DNA samples purified from blood, amniotic fluid, chorionic villus (CVS), or direct sample to PCR using any of the following lysis buffers GT BLB, GT AFLB and GT CVLB.
Along with Duchenne or Becker Muscular Dystrophy detection, the GT HapScreen® DMD kit includes autosomal STR markers used for QF-PCR to detect aneuploidies of chromosomes 21, 18, 13, X and Y as well as many other advantages outlined in the introduction section of GT HapScreen® kits. These markers provide extra advantages along with the detection of diseases.
GT HapScreen® DMDPremium Features
- Aids carrier detection and prenatal diagnosis;
- Shows how disease gene is segregated;
- Rules out/in sample authenticity, maternal cell contamination;
- It aids ruling in/out gonadal mosaicism;
- Chromosomal aneuploidy detection;
- Determination of fetal sex.
For research use only. Not for use in diagnostic procedures
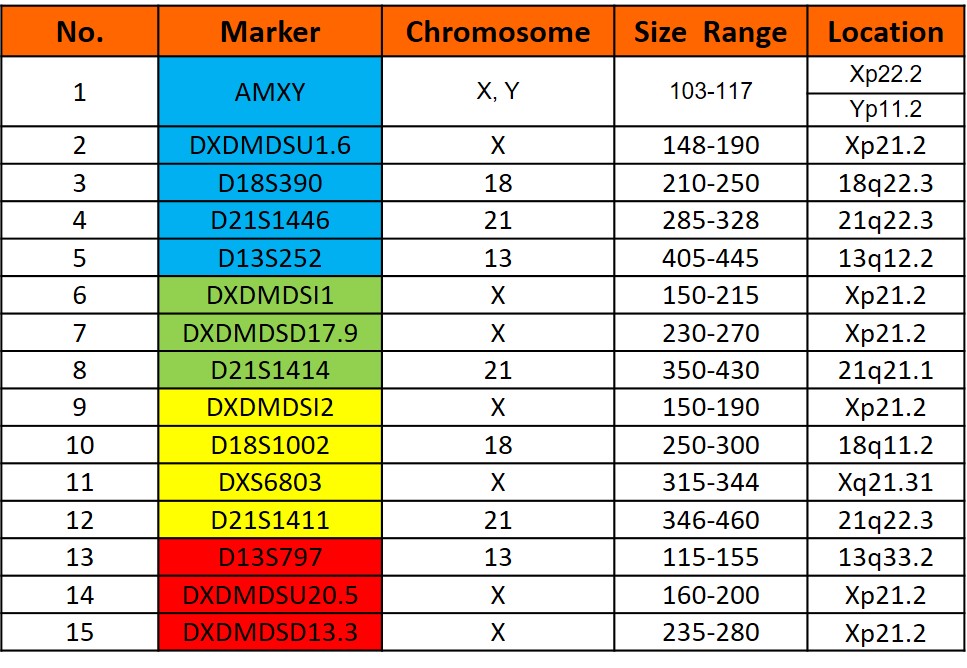
Markers
Markers present in the GT HapScreen® DMD kit
Markers (panel)
DXDMDSU20.5, DXDMDSU1.6, DXDMDSI1, DXDMDSI2, DXDMDSD13.3, DXDMDSD17.9, D21S1446, D21S1414, D21S1411, D18S390, D18S1002, D13S252, D13S797, DXS6803 and AMXY
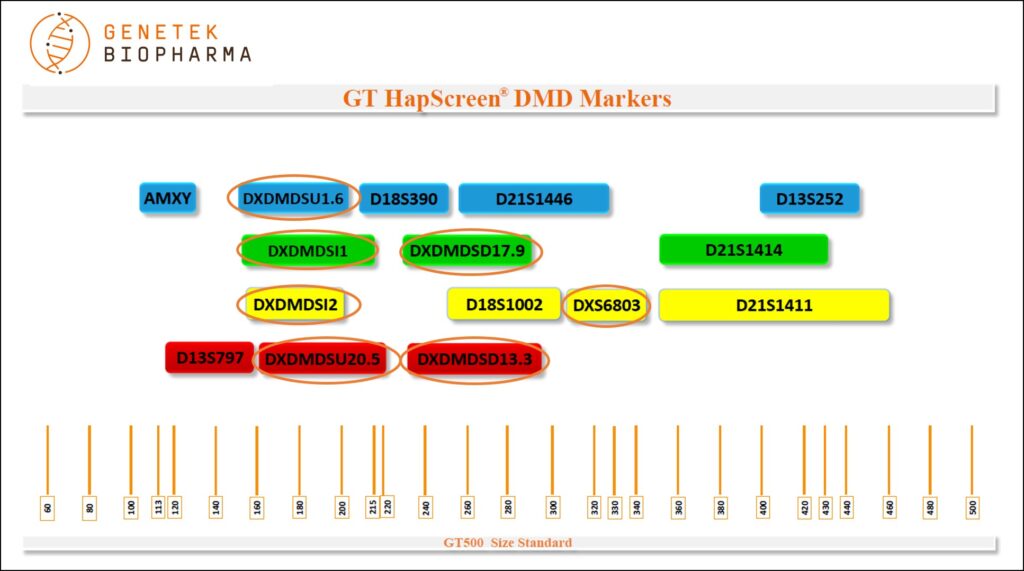
Chromosome Locations

Kit Content
What’s included in the kit
The kit contains all the necessary reagents and buffers for multiplex PCR (Multiplex Primer mix, PCR buffer, GT HSTaq DNA Pol in Box A). Also, the kit comes with GT500 size standard as well as GTM5 Matrix Standard for calibrating the Genetic Analyzer (Box B).